Research
Understanding eukaryotic carbohydrate processing enzymes The Gloster group is interested in understanding eukaryotic carbohydrate processing enzymes from a structural, mechanistic and functional view.
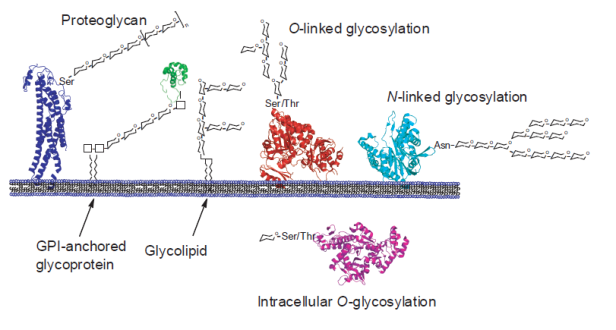
Glycans are ubiquitous biomolecules involved in many diverse cellular processes, including protein folding and localization, cell signalling, organism development and energy storage. They are diverse molecules with huge potential for variation in structure, and can be conjugated to protein or lipids in different ways (see Figure 1). The large chemical diversity displayed by carbohydrates is not defined by a template, but regulated solely by the specificity and localization of the enzymes responsible for their synthesis and degradation.
One of the big challenges in the glycan field is finding the link between the carbohydrate composition in cells, the enzymes that synthesize and degrade it, and the phenotype observed when these enzymes fail. Gaining a basic understanding of enzymes at the molecular level provides a platform on which to design and develop tools in order to probe cellular function. Such probes may also have therapeutic benefit in the longer term.
We use a number of techniques including molecular biology, protein production, X-ray crystallography, enzyme kinetics, and cell culture in order to gain understanding of carbohydrate processing enzymes of interest.
One particular focus is on the enzymes responsible for the degradation of heparan sulphate, a glycosaminoglycan found in the extracellular matrix. The enzymes responsible for completely degrading heparan sulphate are implicated in diseases including lysosomal storage diseases (mucopolysaccharidoses), cancer and neurodegenerative diseases. We aim to investigate these enzymes structurally and mechanistically, and with the development of tools, such as enzyme inhibitors, we are aiming to probe the biological functions of these enzymes.